Dilution and Density of Solutions
Dilution and Density of Solutions
Dilution is process of adding solvent to solution. Since amount of solute stays constant, concentration of solution decreases. We find relation between concentration of solutions before and after dilution with following formula;
M1.V1=M2.V2
Where M1 is initial molarity and M2 is final molarity and V1 and V2 are initial and final volumes of solution.
To increase concentration of solutions, you should add solute or evaporate solvent from solution. Formula given above is also used in increasing concentration of solutions;
M1.V1=M2.V2
Concentration of solutions and volumes are inversely proportional to each other. If volume of solution increases then, molarity of solution decreases. Graph given below shows this relation;

Example: If we add 700 mL water at same temperature to 0,2 molar 300 mL solution, find final molar concentration of this solution.
Solution:
M1=0,2 molar, V1=300=0,3 mL
V2=300+700 =1000mL=1 L
M1.V1=M2.V2
0,2.0,3=M2.1
M2=0,06 molar
Example: If we mix solutions given in the picture below, find concentration of final solution.

Solution: Sum of masses solution one and two gives us mass of final solution
m1+m2=mfinal
80 + 40 =120 g
Sum of solute masses one and two gives us mass of final solute.
(1) msugar1 + msugar2=msugarf
We find masses of solutes by;
msugar1=m1.20/100=80.1/5=16 g
msugar2=m2.30/100=40.30/100=12 g
msugarf=mfinal.X/100=120.X/100 g
we use equation (1) and solve for X;
msugar1 + msugar2=msugarf
16 + 12=120.X/100 g
28=12.X/10
X=23,3
Density of Solutions
We find density of solutions by following formula;
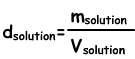
Unit of liquid solutions g/mL or g/cm3. Putting solute into water we prepare solution. When we add solute to solution density of it increases, since increase in the mass of solution is larger than the increase in volume. In solid-liquid solutions, density increases with increasing in the concentration of solution.
Example: Density of H2SO4 solution, having percent by mass 49 %, is 1,2 g/mL. Find molar concentration of this solution. (H2SO4=98)
Solution:
density of solution=1,2 g/mL
Percent by mass= 49 %
Molar mass of H2SO4 is 98 g
We find molar concentration of solution with following formula;
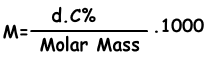
M=(1,2.4)/98 . 1000
M=6 molar
Example: Solubility of X at 15 oC is 20g X/100. Which ones of the following statements are true for solution prepared using 30 g X and 120 g water at 15 oC?
I. Solution is saturated.
II. Mass of solution is 150 g.
III. Concentration percent by mass is 20 %
Solution:
I. at 15oC
100 g water dissolves 20 g X
120 g water dissolves ? g X
¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯
?=24 g X is dissolved.
since 30 g X is added to 120 g water, solution is saturated and 30-24=6 g X stays undissolved. I is true.
II. Mass of solution is equal to sum of solute and solvent.
m=msolute+msolvent
m=120+24=144
Thus mass is not equal to 150 g, II is false.
III. Since 100 g water dissolves 20 g X, there are also 20 g X in 120 g solution. Thus, percent by mass;
X %=(mX/msolution).100
X %=(20/120).100=16,7
III is false