Periodic Table
Periodic Table
Periodic table is prepared for classify elements according to their similarities in chemical and physical properties. In this table, elements are ordered to increasing atomic number. General shape of periodic table is given below.

In periodic table, you can see atomic number, name, symbol and mass number of elements. As you can see from the picture given above, horizontal rows are called period and vertical columns are called group .There are 7 periods and two groups A and B in periodic table. Groups A and B are also have 8 sub groups (8B has three columns). In a period properties of elements change from left to right. In a group, elements have similar chemical properties.
Orbitals in Periodic Table
s block: This blocks contains elements having valence electrons in s orbital. IA and IIA are s block groups. For example,
1s22s22p63s1 and 1s22s2 are s block elements.
p block: This blocks contains elements having valence electrons in p orbitals. IIIA, IVA, VA, VIA, VIIA and VIII A are p block groups. For example,
1s22s22p63s23p5and 1s22s22p63s23p64s23d104p3 are p block elements.
d block: This blocks contains elements having valence electrons in d orbitals. IIIB, IVB, VB, VIB, VIIB, VIIIB, IB and IIB are d block groups. Two elements at the left bottom do not belong to d block. For example,
1s22s22p63s23p64s23d4and 1s22s22p63s23p64s23d10 are d block elements.
d block elements are also called transition elements and all of them are metal.
f block: This blocks contains elements having valence electrons in f orbitals. Two elements mentioned in d block (IIIB) and two rows drawn at the bottom of periodic table are belong to f block.
1s22s22p63s23p64s23d104p65s24d105p66s24f3 is an example of f block element.
f block elements are also called inner-transition elements. They are divided into two groups lanthanides and actinides.
Following periodic table show blocks in detail.
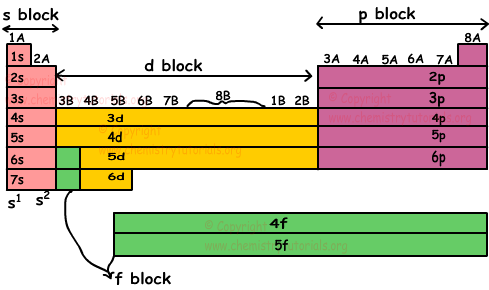
Lanthanides are elements having atomic number between 58 and 71. Actinides are elements having atomic number between 90 and 103.
s and p blocks are called main groups . List given below shows some important group names;
IA=Alkali Metals
IIA=Alkaline Earths
VIIA=Halogens
VIII=Noble Gases