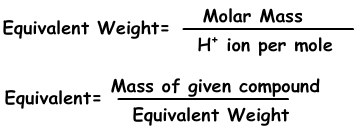Concentratie
Concentratie
Concentratie is de hoeveelheid opgeloste stof in de gegeven oplossing. We kunnen concentratie op verschillende manieren uitdrukken, zoals concentratie in procenten of in mol.
1) Concentratie per procent:
Het is de hoeveelheid opgeloste stof die in 100 g oplosmiddel oplost. Als de concentratie van de oplossing 20% is, begrijpen we dat er 20 g opgeloste stof is in 100 g oplossing. 
Voorbeeld: 10 g zout en 70 g water worden gemengd en de oplossing wordt bereid. Zoek de concentratie van de oplossing op massapercentage.
Oplossing:
Massa opgeloste stof: 10 g
Massa van oplossing: 10 + 70 = 80 g
80 g oplossing bevat 10 g opgeloste stof
100 g oplossing bevat X g opgeloste stof
-————————————————–
X=12,5 g %
Of met behulp van formule;
Massapercentage = 10.100 / 80 = 12,5%
Voorbeeld: Als de concentratie in massa van 600 g NaCl oplossing 40% is, zoek dan de hoeveelheid opgeloste massa in deze oplossing.
Oplossing:
100 g oplossing bevat 40 g opgeloste stof
600 g oplossing bevat X g opgeloste stof
-————————————————–
X = 240 g NaCl zout lost op in oplossing.
Voorbeeld: Als we 68 g suiker en 272 g water toevoegen aan 160 g oplossing met een concentratie van 20%, vind dan de eindconcentratie van deze oplossing.
Oplossing:
De massa van de oplossing is 160 g vóór toevoeging van suiker en water.
100 g oplossing bevat 20 g suiker
160 g oplossing bevat X g suiker
-—————————————-
X = 32 g suiker
Massa opgeloste stof na toevoeging = 32 + 68 = 100 g suiker
Massa oplossing na toevoeging = 272 +68 + 160 = 500 g
500 g oplossing bevat 100 g suiker
100 g oplossing bevat X g suiker
-—————————————–
X = 20% is de concentratie van de uiteindelijke oplossing.
2) Concentratie per Mol:
We kunnen concentratie van oplossingen door mollen uitdrukken. Aantal mol per liter wordt molariteit genoemd, getoond met M. 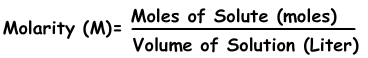
Voorbeeld: Met behulp van 16 g NaOH wordt 200 ml oplossing bereid. Welke van de volgende beweringen zijn waar voor deze oplossing? (Molaire massa van NaOH is 40 g)
I. Concentratie van de oplossing is 2 molair
II. Het volume van het water in oplossing is 200 ml
III. Als we water aan de oplossing toevoegen, neemt het aantal opgeloste stof af.
Oplossing: mol NaOH
I. nNaOH = 16/40 = 0,4 mol
V = 200 ml = 0,2 liter
Molariteit = 0,4 / 0,2 = 2 molair
I is waar
II. Omdat het volume van de oplossing 200 ml is, is het volume water kleiner dan 200 ml. II is fout.
III. Als we water aan de oplossing toevoegen, neemt het volume van de oplossing toe, maar het aantal mol opgeloste stof verandert niet.
Voorbeeld: 4,4 g XCl2 zout lost op in water en vormt 100 ml 0,4 molaire XCl2 oplossing. Vind molaire massa van X. (Cl = 35)
Oplossing:
Molariteit = n / V n=M.V waarbij V=100mL=0,1 L en M=0,4 molair
n=0,1.0,4=0,04 mol
If 0,04 mol XCl2 is 4,4 g
1 mol XCl2 is ? g
-——————————
?=110 g XCl2
Molaire massa van XCl2=X+2.(35)=110
X=40 g/mol
3) Molaliteit:
Molaliteit is de andere uitdrukking van concentratie van oplossingen. Het wordt aangeduid met “m” en de formule van molaliteit is;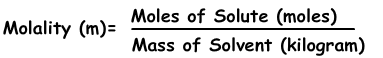
4) Normaliteit:
We kunnen concentratie op een andere manier uitdrukken met normaliteit met behulp van equivalenten van opgeloste stoffen. 
Equivalenten kunnen worden gedefinieerd als; aantal mol H+ ion in zuren en OH- ion in basereacties. Bijvoorbeeld; 1 mol H2SO4 geeft 2 H+ ion, equivalent van H2SO4 is 2. We vinden een equivalent gewicht;